At the smallest scales, the universe behaves in strikingly different ways from the world we observe in our everyday lives. Quantum Mechanics is the branch of physics that describes the peculiar behaviors of microscopic particles such as atoms, electrons, photons, and almost everything else in the molecular and sub-molecular realms.
Table of Contents
The Distinction Between Quantum Mechanics and Classical Physics
Developed during the first half of the 20th century, quantum mechanics presents results that are often strange and counterintuitive. Despite this, studying quantum mechanics has enabled physicists to gain a deeper understanding of the universe’s nature and has the potential to revolutionize the way we process information.
At the scale of atoms and electrons, many equations of classical mechanics—which describe the movement and interactions of objects at everyday sizes and speeds—become inadequate. In classical mechanics, objects are located at specific places at specific times. In contrast, quantum mechanics describes objects as existing in a haze of probability, with a certain chance of being at point A, another chance of being at point B, and so on.
The Origins of Quantum Mechanics
Unlike Albert Einstein’s famous theory of relativity, the origins of quantum mechanics cannot be attributed to a single scientist. Instead, it emerged through the contributions of multiple scientists and gradually gained acceptance and experimental verification between the late 1800s and 1930, as noted by the University of St. Andrews in Scotland.
In 1900, German physicist Max Planck sought to explain why objects at specific temperatures, such as the 1,470-degree-Fahrenheit (800 degrees Celsius) filament of a light bulb, glowed a specific color—red, in this case, according to the Perimeter Institute. Planck realized that equations used by physicist Ludwig Boltzmann to describe gas behavior could be adapted to explain the relationship between temperature and color. However, Boltzmann’s work relied on the concept that gases were composed of tiny particles, implying that light, too, was made of discrete bits.
This idea contradicted the prevalent belief at the time that light was a continuous wave. Although Planck himself didn’t believe in atoms or discrete bits of light, his concept gained traction in 1905 when Einstein published a paper titled “Concerning a Heuristic Point of View Toward the Emission and Transformation of Light.” Einstein proposed that light traveled not as a wave but as “energy quanta,” suggesting that these packets of energy could only be absorbed or generated as wholes, particularly when an atom “jumps” between quantized vibration rates. This notion introduced the “quantum” aspect of quantum mechanics.
Einstein’s perspective on light provided insights into the behavior of nine phenomena, including the specific colors emitted by a light bulb filament described by Planck. It also explained the photoelectric effect, where certain colors of light can eject electrons from metal surfaces.
Wave-Particle Duality
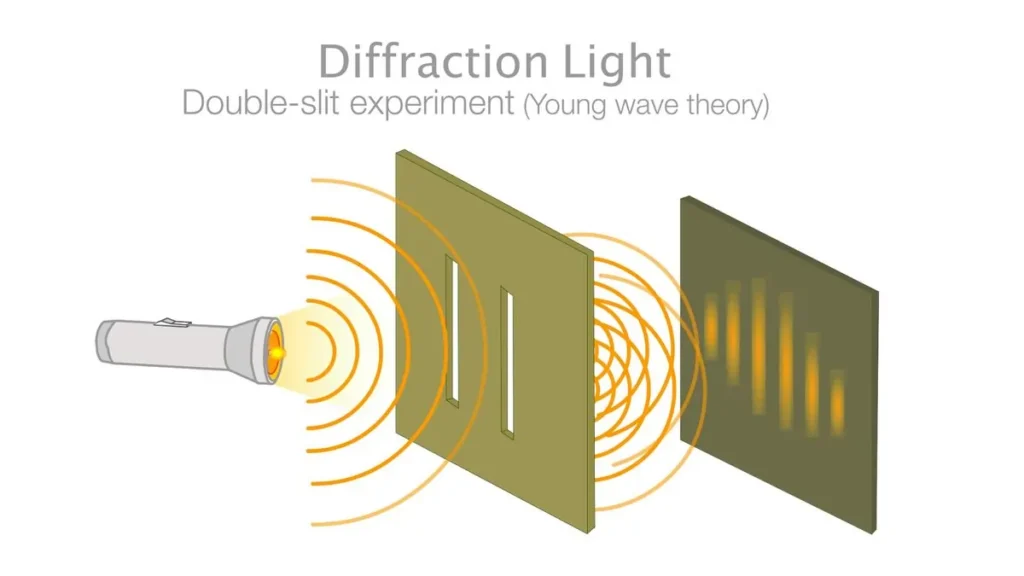
In quantum mechanics, particles can exhibit both wave-like and particle-like properties. This duality is famously demonstrated in the double-slit experiment, where particles such as electrons are shot at a board with two slits, behind which a screen lights up upon electron impact. If the electrons were particles, they would form two bright lines on the screen. However, the experiment reveals an interference pattern of dark and bright bands, which only makes sense if the electrons act as waves with crests and troughs that interfere with each other. Even when a single electron is shot through the slits at a time, the interference pattern emerges, suggesting a single electron can interfere with itself.
In 1924, French physicist Louis de Broglie used Einstein’s special relativity equations to show that particles could exhibit wave-like characteristics and vice versa. This discovery earned him the Nobel Prize a few years later.
Describing Atoms with Quantum Mechanics
In the 1910s, Danish physicist Niels Bohr applied quantum mechanics to describe the internal structure of atoms. By then, it was known that atoms consisted of a heavy, positively charged nucleus surrounded by light, negatively charged electrons. Bohr proposed that electrons orbited the nucleus in predefined distances, receiving or emitting radiation at specific energies by jumping between orbits, reflecting their quantum nature.
Soon after, two scientists independently developed more comprehensive quantum models of the atom. German physicist Werner Heisenberg developed “matrix mechanics,” while Austrian-Irish physicist Erwin Schrödinger created “wave mechanics.” In 1926, Schrödinger demonstrated that these approaches were equivalent. The resulting Heisenberg-Schrödinger model replaced the Bohr model, describing electrons as waves around the nucleus. Electrons obey a “wave function” and occupy “orbitals” with various shapes, as explained by chemist Jim Clark.
Schrödinger’s Cat Paradox
Schrödinger’s Cat is a famous thought experiment illustrating some early quantum mechanics developers’ discomfort with its implications. While Bohr and his followers believed quantum mechanics suggested particles lack well-defined properties until observed, Schrödinger and Einstein found this idea absurd. In 1935, Schrödinger proposed an experiment where a cat’s life depended on a quantum particle’s random state, remaining unknown until the box was opened. According to Bohr’s interpretation, until the box was opened, the cat existed in both alive and dead states simultaneously. Schrödinger and Einstein used this paradox to argue that quantum mechanics was incomplete and would eventually be replaced by a more intuitive theory.
Quantum Entanglement
Einstein, Schrödinger, and others highlighted another peculiar quantum mechanics result: entanglement. In 1935, Einstein, Boris Podolsky, and Nathan Rosen showed that two quantum particles could be correlated so that measuring one particle’s state instantly reveals the other’s state, regardless of distance. Einstein called this “spooky action at a distance,” while Schrödinger coined the term “entanglement.” This phenomenon is now recognized as a fundamental aspect of quantum mechanics and underpins the emerging field of quantum computing.
Quantum Computing
Quantum computing leverages the unique properties of quantum mechanics. Unlike classical computers that use binary bits (0 or 1), quantum computers use qubits, which can represent both 0 and 1 simultaneously due to superposition. This allows quantum computers to perform parallel calculations. Additionally, quantum entanglement enables qubits to share information instantaneously.
Despite their potential, quantum computers currently face challenges such as small size, maintenance difficulty, and high error rates. Nevertheless, experts believe these issues will be overcome as the field advances.
The Incompatibility of Quantum Mechanics and General Relativity
Physicists have yet to find a comprehensive theory that explains all observed particles and forces in the universe. Einstein’s relativity describes large, massive objects, while quantum mechanics describes small, insubstantial ones. Though not exactly incompatible, these theories have yet to be unified.
Researchers have proposed various theories of quantum gravity, which aim to incorporate gravity into quantum mechanics and explain phenomena from the subatomic to the cosmic scale. Proposals include the hypothetical graviton and string theory, which posits that fundamental entities are tiny vibrating strings in multiple dimensions. However, string theory has lost some favor due to a lack of supporting evidence. Other proposals, such as loop quantum gravity, suggest that time and space consist of discrete chunks. Despite these efforts, no single theory has achieved widespread acceptance in the physics community.
Quantum mechanics is a fundamental theory in physics that describes the behavior of matter and energy at the smallest scales, such as atoms and subatomic particles. Here are 20 frequently asked questions (FAQs) about quantum mechanics, along with detailed answers:
Frequently Asks Questions (FAQs)
What is Quantum Mechanics?
Quantum mechanics is a branch of physics that deals with the behavior and interactions of particles at the atomic and subatomic levels. It introduces the concept of quantization, wave-particle duality, uncertainty principles, and the probabilistic nature of physical phenomena. Unlike classical mechanics, which describes macroscopic objects, quantum mechanics is essential for understanding the behavior of particles like electrons, protons, and photons.
What is the Wave-Particle Duality?
Wave-particle duality is a fundamental concept in quantum mechanics, stating that particles such as electrons and photons exhibit both wave-like and particle-like properties. This dual nature is evident in phenomena like the double-slit experiment, where particles create an interference pattern, indicative of wave behavior, while also being detected as discrete particles.
What is the Heisenberg Uncertainty Principle?
The Heisenberg Uncertainty Principle states that it is impossible to simultaneously measure both the position and momentum of a particle with absolute precision. The more accurately one of these properties is measured, the less accurately the other can be known. This principle reflects a fundamental limit to measurement in quantum systems and highlights the probabilistic nature of quantum mechanics.
What is a Quantum State?
A quantum state is a mathematical object that contains all the information about a system’s properties. It is represented by a wave function, denoted by the Greek letter Ψ (Psi). The wave function describes the probability amplitude of finding a particle in a particular state, and the square of its absolute value gives the probability density.
What is Quantum Superposition?
Quantum superposition is the principle that a quantum system can exist in multiple states simultaneously. A particle can be in a combination of different states, and only upon measurement does it ‘collapse’ into one of the possible states. This concept is illustrated by Schrödinger’s cat thought experiment, where a cat in a box can be simultaneously alive and dead until observed.
What is Quantum Entanglement?
Quantum entanglement is a phenomenon where two or more particles become interconnected such that the state of one particle instantaneously influences the state of the other, no matter how far apart they are. This connection persists even if the particles are separated by vast distances. Entanglement challenges classical intuitions about locality and causality and plays a crucial role in quantum computing and quantum communication.
What is the Schrödinger Equation?
The Schrödinger Equation is a fundamental equation in quantum mechanics that describes how the quantum state of a physical system evolves. It is a wave equation that governs the behavior of wavefunctions. The time-dependent Schrödinger Equation provides a way to predict the future state of a system based on its current state, while the time-independent version is used for systems in a steady state.
What is Quantum Tunneling?
Quantum tunneling is a quantum mechanical phenomenon where particles have a probability of passing through a potential barrier, even if they do not have enough energy to do so classically. This effect is a result of the wave-like nature of particles and is essential in various processes, such as nuclear fusion in stars and the operation of semiconductor devices.
What is the Copenhagen Interpretation?
The Copenhagen Interpretation is one of the oldest and most widely taught interpretations of quantum mechanics. It posits that physical systems do not have definite properties until measured. The act of measurement causes the wavefunction to collapse, resulting in a specific outcome. This interpretation emphasizes the probabilistic nature of quantum mechanics and the role of the observer.
What are Quantum Numbers?
Quantum numbers are sets of numerical values that describe the quantum state of a particle. They include the principal quantum number (n), which indicates the energy level; the angular momentum quantum number (l), which describes the shape of the orbital; the magnetic quantum number (m_l), which specifies the orientation of the orbital; and the spin quantum number (m_s), which represents the intrinsic spin of the particle.
What is Quantum Decoherence?
Quantum decoherence is the process by which a quantum system loses its quantum properties, such as superposition and entanglement, due to interaction with its environment. This interaction causes the system to transition from a coherent superposition of states to a classical mixture of states, effectively destroying quantum interference effects. Decoherence is a significant challenge in quantum computing and information processing.
What is the Many-Worlds Interpretation?
The Many-Worlds Interpretation suggests that all possible outcomes of quantum measurements occur, each in a separate, parallel universe. According to this view, the universe splits into multiple copies whenever a quantum event with multiple possible outcomes occurs. This interpretation avoids the concept of wavefunction collapse and instead postulates that all branches of the wavefunction continue to exist.
What is the Quantum Measurement Problem?
The Quantum Measurement Problem refers to the question of how and why the process of measurement causes a quantum system to ‘collapse’ from a superposition of states into a single state. This problem is central to the interpretation of quantum mechanics and raises questions about the nature of reality, the role of the observer, and the completeness of the theory.
What is Quantum Field Theory?
Quantum Field Theory (QFT) is a theoretical framework that combines quantum mechanics with special relativity. It describes particles as excitations of underlying fields that permeate space and time. QFT is the foundation for understanding the behavior of fundamental forces and particles, including the electromagnetic, weak, and strong interactions, as well as the Higgs boson.
What is the Standard Model of Particle Physics?
The Standard Model of Particle Physics is a theory that describes the fundamental particles and forces in the universe, except for gravity. It includes three families of elementary particles: quarks, leptons, and gauge bosons, which mediate the strong, weak, and electromagnetic forces. The discovery of the Higgs boson in 2012 provided the last missing piece of the Standard Model.
What is Quantum Computing?
Quantum computing is a field of study focused on developing computers based on the principles of quantum mechanics. Unlike classical computers, which use bits to represent information as 0s or 1s, quantum computers use quantum bits, or qubits, which can be in a superposition of states. This allows quantum computers to solve certain problems exponentially faster than classical computers.
What are Quantum Cryptography and Quantum Communication?
Quantum cryptography and quantum communication use the principles of quantum mechanics to secure communication channels and protect information. One of the most well-known applications is Quantum Key Distribution (QKD), which allows two parties to securely share a cryptographic key. The security of QKD is based on the principles of quantum superposition and entanglement, which ensure that any eavesdropping attempt will be detected.
What is Bell’s Theorem?
Bell’s Theorem is a fundamental result in quantum mechanics that shows that no physical theory of local hidden variables can reproduce all the predictions of quantum mechanics. This theorem, proposed by physicist John S. Bell, provides a way to test the concept of local realism—the idea that physical processes are not influenced by distant events. Experiments testing Bell’s inequalities have confirmed the nonlocal nature of quantum entanglement.
What is the EPR Paradox?
The EPR Paradox, named after Einstein, Podolsky, and Rosen, is a thought experiment that challenges the completeness of quantum mechanics. It questions the nature of reality and locality, suggesting that quantum mechanics might allow “spooky action at a distance.” The paradox highlights the strange and non-intuitive nature of entanglement, where the measurement of one particle seems to instantaneously affect the state of another, distant particle.
How Does Quantum Mechanics Differ from Classical Physics?
Quantum mechanics differs from classical physics in several key ways:
- Quantization: Energy levels are discrete in quantum systems, unlike the continuous spectra in classical systems.
- Wave-Particle Duality: Particles exhibit both wave-like and particle-like properties.
- Uncertainty Principle: There are fundamental limits to how precisely certain pairs of properties (like position and momentum) can be known.
- Superposition and Entanglement: Quantum systems can exist in multiple states simultaneously and can be entangled, exhibiting correlations that cannot be explained classically.
- Probabilistic Nature: Predictions in quantum mechanics are inherently probabilistic, in contrast to the deterministic predictions of classical mechanics.
Read More Articles
- Unraveling the Mysteries of Dark Matter and Dark Energy
- Exploring the Quantum Revolution: Unveiling the Power of Quantum Physics
- The F-22A Raptor: A Comprehensive Overview
- Why Cyber Security is Essential: Exploring 5 Key Reasons
- Astra Space’s Financial Struggles: A Close Brush with Bankruptcy
- Introducing the Xogdor Rocket to the Military Realm
- The Formation of the LEO Owner Operators Affinity Group
- Expanding Satellite-to-Smartphone Connectivity
- Navigating Privacy and Safety in Adult Webcam Streaming
- Choosing Between Open Source and Proprietary LLMs
- Rethinking Food Packaging for a Sustainable Future
- Mastering Typography in Web Design
- Exploring Typography Trends for Digital Design in 2024
- Unlocking Sustainable Economic Growth Through Innovation
- Navigating the Financial Landscape of Executive Education and MBA Programs
- Understanding Information Technology: The Backbone of Modern Business
- Understanding Algorithms: Definition, Functionality, and Real-Life Applications
- Understanding Machine Learning: A Comprehensive Exploration
- Unlocking the Power of Machine Vision: Revolutionizing Industries
- Unlocking the Power of Expert Systems: Enhancing Decision-Making with AI
- Unlocking the Power of Natural Language Processing
- Unlocking the Secrets of Artificial Intelligence
- Optimizing Logistics with AI: Revolutionizing Efficiency in Package Routing


